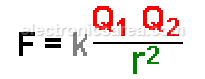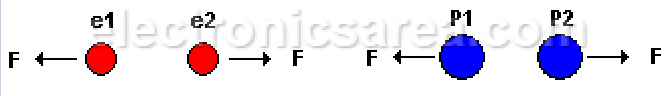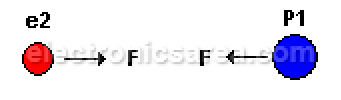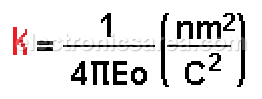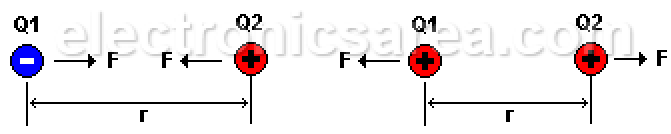The electrostatic force that acts on one point charge q 1 due to the presence of another point charge q 2 is given in the Coulomb’s Law:
Electrostatic Force
All objects are made of atoms. Atoms are made up of electrons, protons, and neutrons. Electrons, protons, and neutrons have mass, but only the electron and the proton have charge. The proton has a positive charge and the electron has a negative charge.
If we place two electrons (both negatively charged) at an “r” distance, they will repel each other with a “F” force. This force depends on the “r” distance between the electrons and the charge of each. This “F” force is called the electrostatic force.
If we use protons instead of electrons, there is also a repulsion force because the charges are the same. (both positive). The force changes to attractive if instead of two elements with equal charges, we use two elements with different charges. (one electron and one proton).
Electrostatic force is a force of attraction or repulsion, depending on the type of charges:
- Negative charge (e1) repels negative charge (e2).
- Positive charge (P1) repels positive charge (P2).
- Positive charge (P1) with negative charge (e2) attract each other.
- An electron (e) with a neutron (N) produces no force.
- A proton (P) with a neutron (N) produces no force.
Remember that the neutron (N) is “neutral”, it has no charge. In conclusion:
Charges of the same type repel each other,
different charges attract each other.
Note: Protons and electrons have equal but opposite charges. However, the mass of the proton is nearly 2000 times that of the electron.
What is Coulomb’s Law?
The electrostatic force depends on the opposing electric charges and the distance between them. The value of this electrostatic force is given by Coulomb’s Law.
Where:
- F = electrostatic force acting on each charge Q1 and Q2.
- K = a constant that depends on the unit system and the environment in which the charges are located.
- r = distance between the charges.
In the MKS system and in a vacuum environment:
Where
In the MKS system: k = 9 x 109 Newton-meter2/coulomb2
With this value of k, the charges are expressed in coulombs and the distance (r) is expressed in meters to give a resultant force in newtons.
- If the charges are opposite (+ and -), F will be negative, indicating attraction.
- If the charges are equal (+ and + or – and -), F will be positive, indicating repulsion.
Note: The units of the MKS system are: (meters, kilograms, seconds).
Example: How to calculate the force between 2 charges using Coulomb’s law
Two charges q1 = 8 x 10-6 C and q2 = 7 x 10-6 C (both positive charges) are 0.0005 meters apart. What is the electrostatic force between them?
We have:
- q1 = 8 x 10-6 Coulombs
- q2 = 7 x10-6 Coulombs
- r = 0.0005 meters
- k = 9 x 109 Newton-meter2/coulomb2
Using Coulomb’s law formula: F = k (q1 . q2)/r2, we get:
F = (9 x 109 Newton-meter2/coulomb2 ) . ( 8 x 10-6 Coulombs) . ( 7 x10-6 Coulombs) / (0.0005 meters)2
F = (9 x 109) (8 x 10-6) (7 x10-6) / (0.0005)2 = 2.013211648 x 106 Newtons. (F is a force of repulsion).