The structure of matter
The matter is divided into molecules, which in turn are divided into atoms. These atoms are composed of two parts: nucleus and periphery.
- 1 – In the nucleus of the atom there are positive charged protons, neutrons which, as its name suggests, have no electric charge or are neutral.
- 2 – On the periphery of the atom, there are negatively charged electrons.
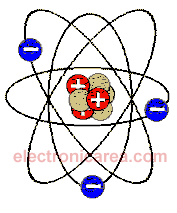
Bohr atom
The Danish physicist, Niels Bohr, created the model (later called Bohr model), where the structure of the atom is shown. See the diagram below.
The number of electrons (in blue color) equals the number of protons (in red color), so we say that the atom is electrically neutral. # Proton = # Electron. There are some electrons in the outer orbits of the atom and are the furthest from the core. These electrons can be released from the attraction of the nucleus more easily and are called valence electrons.
Example: The copper atom has 29 protons and 29 electrons. This atom has 28 electrons in orbits close to the nucleus and 1 electron is in a distant orbit. This electron is called: free electron or valence electron.
- If a material has many free electrons, we call it a conductive material.
- If a material has few free electrons, we call it an insulating material.
Examples:
- Conductive materials: gold, silver, aluminum, copper, etc.
- Insulating materials: ceramics, glass, wood, paper, etc.
When an atom has fewer electrons than normal, it is called a positive ion. When an atom has more electrons than normal, it is called a negative ion.
Electricity
Electricity is the accumulation or movement of electrons, which have been removed from their orbits. (see above). These electrons are called free electrons that, when they are taken out of their orbits, can move easily through a material. This is called electric current.