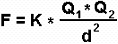Electric charge, the atom, positive Ion and negative Ion
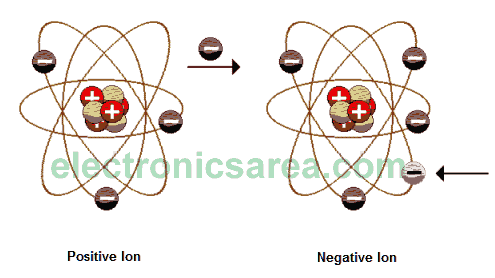
The atom, in normal conditions, is neutral (electrically speaking), this is because, in any atom, the number of protons equals the number of electrons. Using different methods (rub, induction, etc. …) we can make the atom loses or gains electrons.
Thus, the atom is not neutral anymore and has a positive or negative charge respectively. A electrically charged atom is called ION.
Because of the neutral characteristic of the atom, we know that the proton and electron have the same electric charge but different sign. We can therefore consider the electron as the elementary unit of the electric charge. One of the fundamental principles of electrostatics states that:
“Two charges with the same signs repel each other, while two charges with different signs attract”
The power of attraction or repulsion of two electric charges is obtained using the Coulomb’s law, which says:
“The force of attraction or repulsion between two bodies, is directly proportional to the electric charges that they both possess and inversely proportional to the square of the distance that separates them”.
- F = Force of attraction
- Q = Charge of the body
- d = Distance
As the attractive force of the nucleus on the electrons, due to Coulomb’s law, it is lower the further away the orbit is. The atom share or take electrons only in its last orbit. Electrons of the last orbit are ready to leave this orbit. Electrons of the last orbit are called valence electrons.
We have said that the electron (or proton, since they have the same charge) is the basic unit of electric charge. This charge unit is very small, because in one centimeter of any material there are millions of electrons. To work with electrical charges, the coulomb is used as a measurement unit.